ANSI/AAMI/IEC 62366
Application of Human Factors for Medical Devices
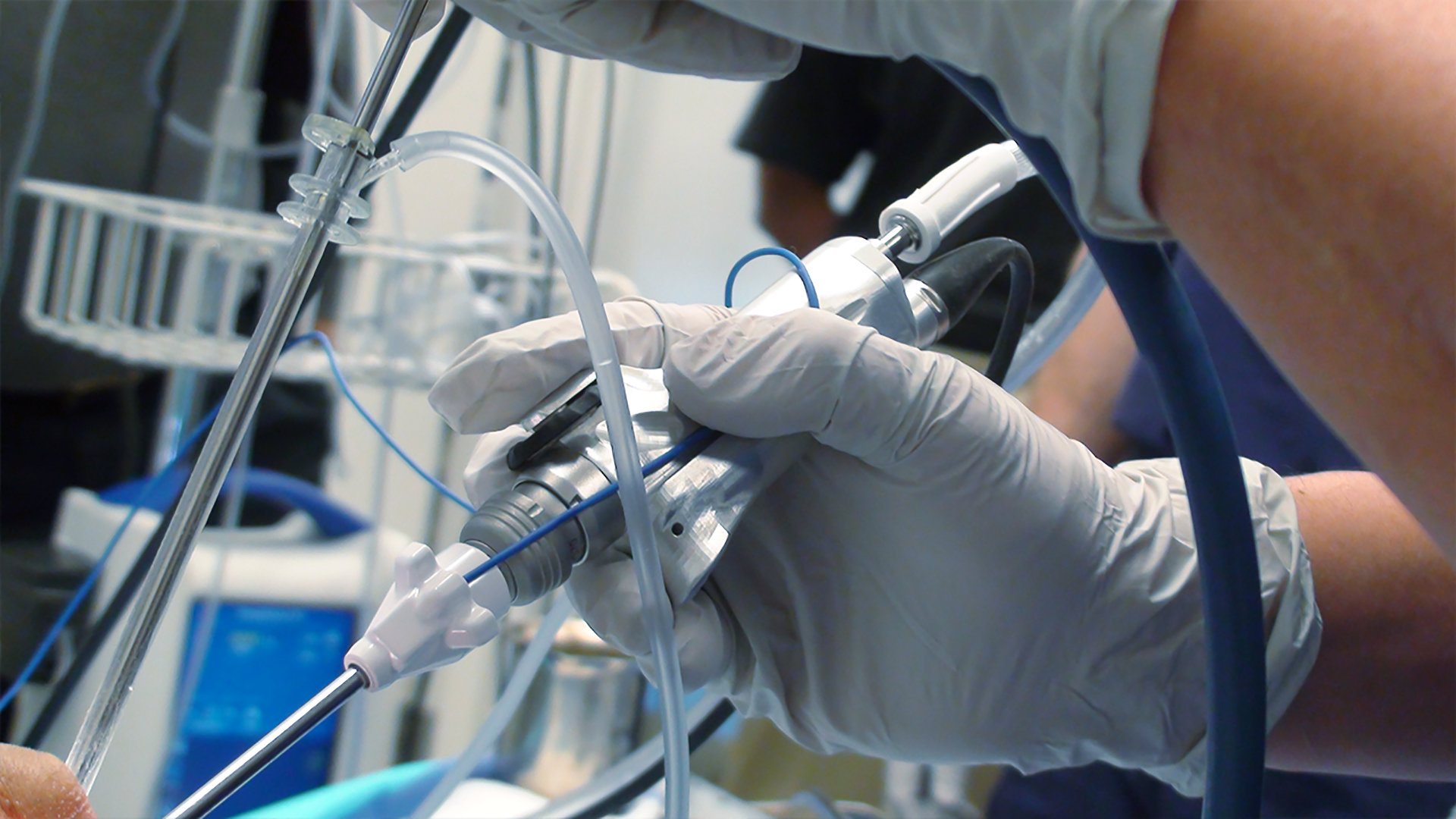
Optimal usability engineering emphasizes a user-centered approach throughout the medical product design and development process.
This has been central to Metaphase’s best practices since we opened our doors in 1991. The user-centered approach is strongly emphasized in human factors engineering guidance documents ANSI/AAMI HE75 Human Factors Principles for Design of Medical Devices and IEC 62366 Application of Usability Engineering to Medical Devices. Specifically, HE75 targets a broad range of recommended usability engineering, user-centered, best-practices for human factors engineering while 62366 focuses on use safety. Following 62366 guidance ensures verification/formative and validation/summative studies align with both the FDA’s usability engineering requirements as well as the requirements of the European Union Medical Device Regulation (MDR) entities.
• Competitive Product Benchmarking
• Research Protocol and Methodology Development
• Generative Studies
• Formative and Summative Usability Evaluation Studies
• Expert User Interface Evaluation
• Ergonomic and Design Audits
• Task Analysis
• Threshold Analysis
• Risk Management Principles
• Contextual Inquiry Methods
• Heuristic Evaluation
• Study Execution
o IRB Facilitation
o Subject Recruitment
o Research Facility – On Site Observation and Live Streaming

